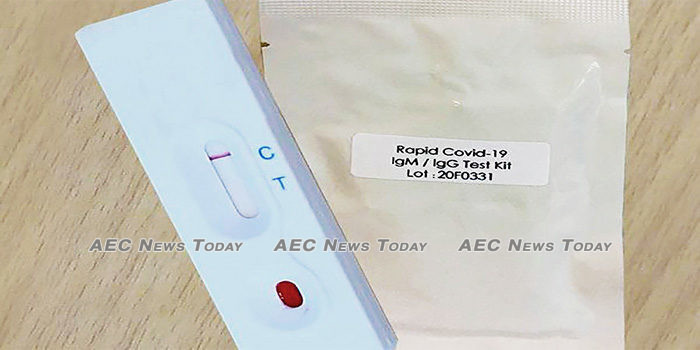
Governments across Asean have raised alarm over the sudden emergence of unregistered COVID-19 instant test kits being sold online and in markets, warning the public not to fall victim to the unproven devices.

Although accurate testing is critical to fast-track diagnosis and reporting of COVID-19, testing in some countries is tightly controlled, a shortage of test kits just one of the reasons.
As anxiety grows profiteers have been quick to step up with what they claim to be COVID-19 instant test kits, able to churn out accurate indications of the presence of SARS-CoV-2 antibodies in a few minutes, for under $50 a test.
In Cambodia, Prime Minister Hun Sen, told a press briefing for local media at the National Assembly (NA) that the results of the test kits and medicines sold in the market are not reliable.
“These [unregistered testing kits] have to be seized immediately. Even if we do not have a law for this action yet, the illegal products must be seized especially since they have not been approved by the World Health Organisation (WHO).
“It has to be done to prevent the people from suffering further during this pandemic”, he said, ordering that those selling the items should be processed in accordance with the Law on Combating Counterfeit Medicines.
In a statement Health Ministry spokesperson, Or Vandine told local media that “the effectiveness of the rapid test kits is very low. People who contracted COVID-19 tested negative, while those who had not contracted the SARS-CoV-2 virus had tested positive. So, these are very dangerous”, the Khmer Times quoted her as saying.
Quick arrests and seizures
| How a nasal swab sample for COVID-19 is taken — not something you can do at home. Video LAU Medical Center-Rizk Hospital |
Cambodian police promptly rounded up 13 people across the country, five of whom were foreign nationals, for producing and selling unauthorised, COVID-19 instant test kits. Doubling-down, Cambodia’s Ministry of Health (MOH) vowed to further regulate the sale of pharmaceutical drugs, medical equipment and disinfectants, including prohibiting the sale of such products online.
In Thailand the Food and Drug Administration (Thai FDA) has issued similar warnings not to buy the so-called ‘Covid-19 instant test kits’ being sold online, saying none have been approved by the agency.
Echoing the FDA’s warnings, the Association of Medical Technologist of Thailand (AMTT) on Monday warned that the use of blood tests at home, rather than in a clinical environment, increases the opportunity for the spread of Covid-19 in addition to other pathogens that can be transmitted through the blood, such as Aids and hepatitis.
In the Philippines, where a shortage of testing kits and government approved laboratories has seen people desperate to test themself as the number of infections and deaths rise, the government has issued similar warnings over the unapproved COVID-19 instant test kits and on March 12 its FDA issued a circular prohibiting the distribution, sale or use of COVID-19 instant test kits without its authorisation.
Philippines FDA Director General, Eric Domingo said that COVID-19 test kits based on real time polymerase chain reaction (RT-PCR) were recommended and warned that suppliers must not import “point-of-care or rapid COVID-19 test kits” before they are certified.
On Monday (March 30) the agency approved five COVID-19 antibody test kits, ramping up the government’s capability to test for the SARS-CoV-2 virus, though samples must still undergo a confirmatory test using the machine-based method. It also approved the use of a PCR-based test kit by Abbott Laboratories, which can detect the SARS-CoV-2 virus within 15 minutes.
Embarrassment for Beijing

After successfully taming the COVID-19 outbreak at home, the dubious quality test kits flooding Asean markets, as well as other countries, is proving an embarrassment for Beijing.
Spain ditched Chinese-made COVID-19 instant test kits intended for mass testing after it found that they only had a sensitivity of 30 per cent, when it should be higher than 80 per cent.
According to the Spanish Society of Infectious Diseases and Clinical Microbiology (SEIMC), nose swabs developed by Shenzhen Bioeasy Biotechnology (SBB) — the producer of the COVID-19 instant test kits — had an accuracy rate of less than 30 per cent. Spanish newspaper El País reported that the Madrid city government had ordered 340,000 test kits from the company.
In light of the adverse reaction the COVID-19 instant test kits are causing globally the Chinese government yesterday (April 1) banned export of test kits and other medical supplies by companies who do not have the licences necessary to sell their products in the local market. The decision is expected to seriously impact local manufacturers who produce products for export markets only, which often do not need local quality certification.
Local instant test kits enter market
While some of the Chinese-made COVID-9 instant test kits are causing concern over their reliability and accuracy, Thailand’s Chulalongkorn University has begun rolled out its “quick and easy” COVID-19 instant strip test. Developed by lecturers at the Faculty of Pharmaceutical Sciences of Chulalongkorn University, the blood-based test is said to be able to detect SARS-CoV-2 antibodies in less than 15 minutes.
Meanwhile, a SARS-CoV-2 PCR Detection Kit developed by scientists from the University of the Philippines National Institute of Health (UP-NIH) and the Philippine Genome Center, with the support of the Department of Science and Technology, is expected to be approved for use this week, with sales expected to commence April 13.
Feature photo Chulalongkorn University
Related:
- Search and seizure for counterfeit test kits (Khmer Times)
- Spanish capital ditches ‘unreliable’ Chinese coronavirus test kits (Yahoo News)
- FDA warns against imported rapid COVID-19 testing kits (CNN Philippines)
Stella-maris Ewudolu
Between November 2010 and February 2012 she was a staff writer at Daylight Online, Nigeria writing on health, fashion, and relationships. From 2010 – 2017 she worked as a freelance screen writer for ‘Nollywood’, Nigeria.
She joined AEC News Today in December 2016.
Latest posts by Stella-maris Ewudolu (see all)
- Zoonotic crossover fear sees Vietnam ban (almost) all wildlife trade (video) – July 26, 2020
- Job & revenue losses: COVID-19 to hurt Asean airlines the most – July 24, 2020
- Philippines morning news for July 24 – July 24, 2020
- Philippines morning news for July 23 – July 23, 2020